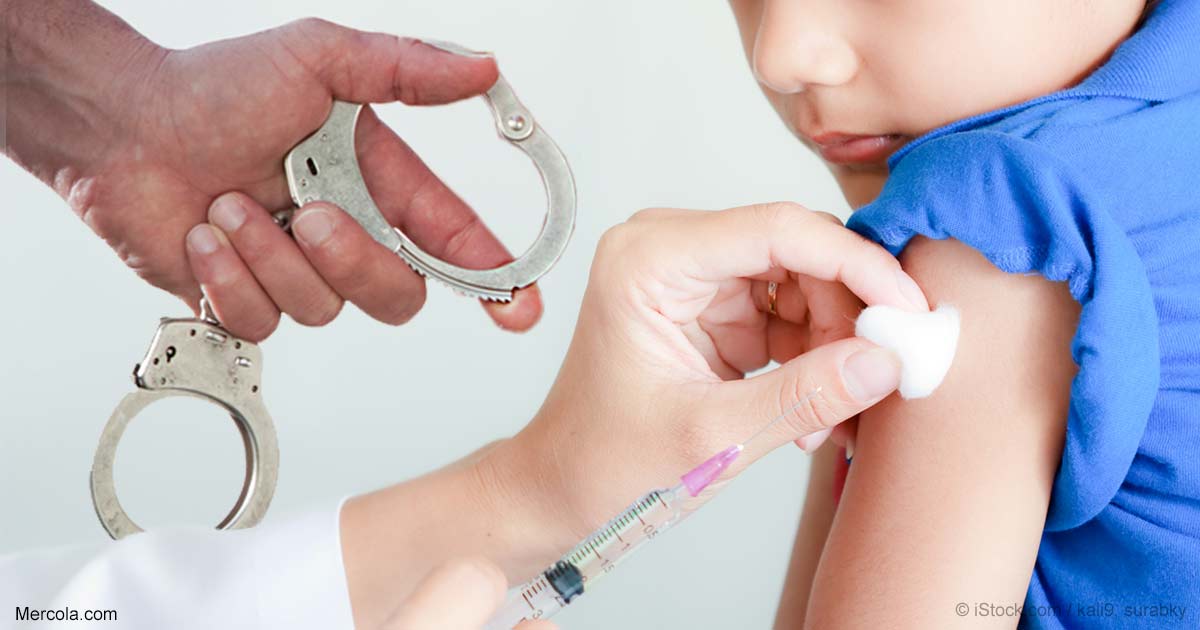
By Dr. Mercola Many people are unaware that in 1986 Congress gave the U.S. vaccine industry a shield from product liability that is unlike any other in existence. In most cases, if a pharmaceutical product injures or kills a person, the manufacturer of that product can be held financially accountable in a civil court of law. With vaccines, however, this is not the case. In the U.S., there is a federally operated vaccine injury compensation program (VICP) that Congress created under the National Childhood Vaccine Injury Act. The VICP was created 30 years ago as an administrative alternative to a lawsuit when federally licensed vaccines recommended for children cause injury or death. Federal compensation was supposed to be awarded when there was no other biologically plausible explanation for the vaccine-related injury or death, and plaintiffs denied federal compensation or offered too little were supposed to be able to access civil courts. However, in 2011, the U.S. Supreme Court effec
http://articles.mercola.com/sites/articles/archive/2017/07/11/vaccine-industry-worried-about-accountability.aspx
No comments:
Post a Comment